X-RAY TECHNOLOGY
X-ray fluorescence (XRF)
X-ray fluorescence (XRF) is a non-destructive analytical method used to determine elemental concentrations in various materials.
XRF works by striking a sample with an x-ray beam, causing characteristic x-rays to fluorescent from each element in the sample. A detector measures the energy and intensity (number of x-rays per second at a specific energy) of each X-ray, which is transformed into an elemental concentration using either a standardless technique such as fundamental parameters or user-generated calibration curves.
The presence of an element is identified by the element’s characteristic X-ray emission wavelength or energy. The amount of an element present is quantified by measuring the intensity of that element’s characteristic X-ray emission.
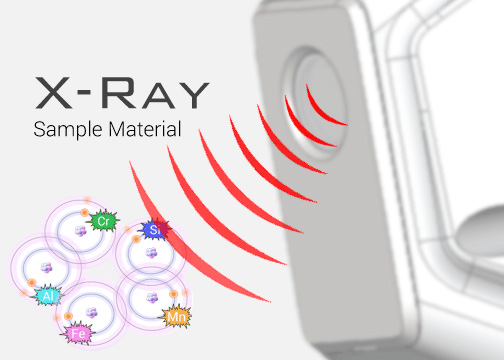
High-energy primary X-ray photons are emitted from an X-ray source and strike the sample.
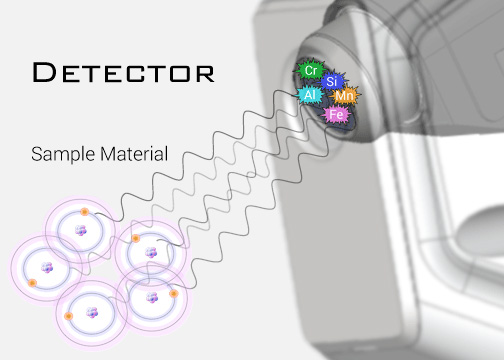
The fluorescent energy is transferred to a detector, where it is absorbed and transferred into an electrical signal and then into a number (digitized).
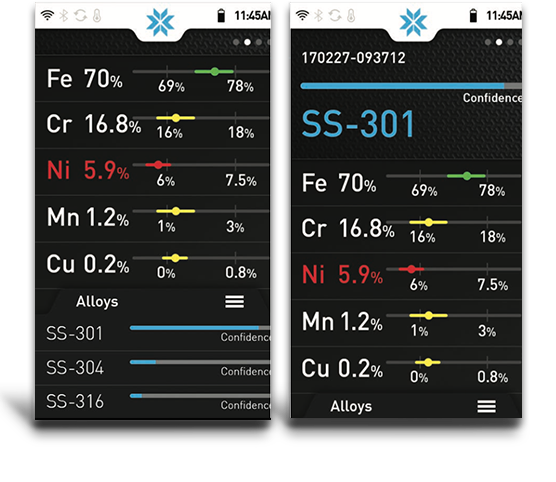
Results
Results can be viewed in the form of percentages, or as spectrum. The XRF will process (digitize, count) about 200,000 or more x-rays every second. These detected x-rays form a spectrum. Each peak in the spectrum is from a characteristic x-ray that was emitted by a specific element, like Cr, or Ni, etc.
The height of the peak is proportional to concentration of the element. The peak height is converted to a percentage or ppm of that element via a calibration method - either fundamental parameters or factory or user-derived empirical calibrations
Benefits of ED-XRF technologie :
- Versatile Testing Equipment, potentially solving multiple analytic needs with one sy
- Non-Destructive Testing Technology
- Almost Instant Results; depending on the application, results in 5-60 seconds
- Relatively Simple Technology; most users can learn how to take measurements quickly
SDD versus Si-PIN Diode
Handheld x-ray fluorescence analyzers for elemental analysis have traditionally been equipped with Si-PIN detectors that provided outstanding resolution and count rate capacity at the time. However, since Silicon Drift Detectors (SDD) were introduced they have become the ‘detector of choice’ for many material analysis applications.
Si-PIN technology offers an economical detection system which, in turn, allows for a lower priced analyzer. They also offer a larger active area and thicker depletion depth. When resolution is critical but high detection efficiency is not important, the Si-PIN system will be suggested. However, SDD technology offers a much better energy resolution at short peak times; which is helpful at high count rates and can lead to lower limits of detection.
Typical SDD Application :
- Ultra-Low Sulfur Identification
- Wear Metals Analysis of Lubrication Oils
- Wood Preservatives Analysis
- Silicone on Paper Testing
- Hazardous Substance Screening (RoHS)
LIBS TECHNOLOGY
Handheld Laser Induced Breakdown Spectroscopy (HH LIBS)
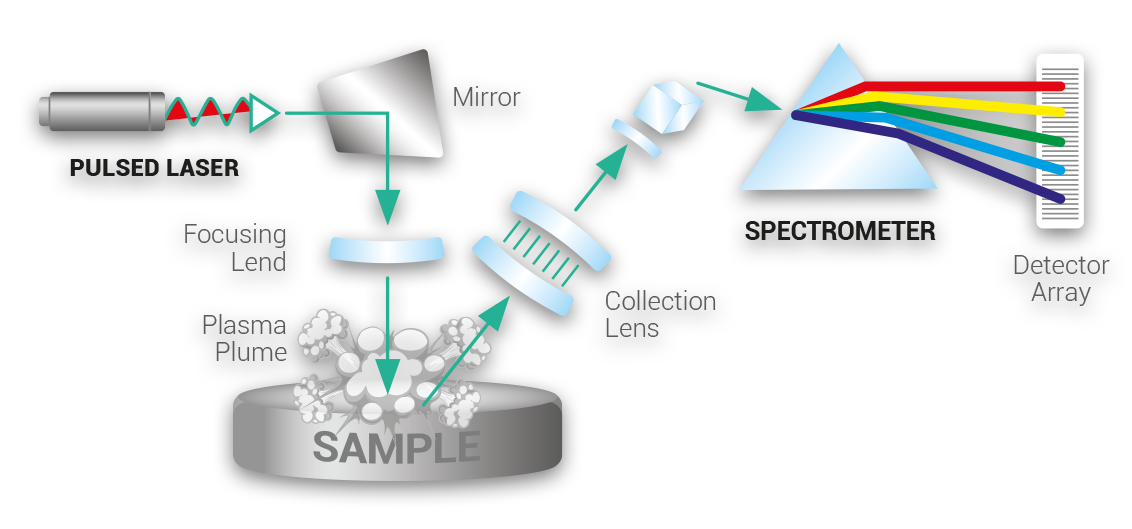
Laser Induced Breakdown Spectroscopy (LIBS) is a rapid chemical analysis technology that uses a short laser pulse to create a micro-plasma on the sample surface.
LIBS is a type of optical emission spectroscopy used to measure elemental concentrations in a material. LIBS operate by using a pulsed, focused laser that is fired at a sample with sufficient pulse energy as to create a plasma around the area struck. Bound atomic electrons are striped from the atoms comprising the material. As the plasma cools, atoms recombine with electrons and in the process, emit light in the UV, optical and IR regimes.
LIBS have been used for more than 30 years as a laboratory technique, capable of analyzing any element in the periodic table. Recently, the technique has been miniaturized into a handheld device (HH LIBS) capable of analyzer any element, depending on the spectrometer range chosen for the device.
LIBS attributes
LIBS have many attributes that make it an attractive tool for chemical analysis. A recent breakthrough in component development, the commercial launching of a small, high-resolution spectrometer, has greatly expanded the utility of LIBS and resulted in a new potential for field-portable broadband LIBS because the technique is now sensitive simultaneously to all chemical elements due to detector response in the 200 to 980 nm range with 0.1 nm spectral resolution.
Advantages
A small size and weight; B technologically mature, inherently rugged, and affordable components; C in-situ analysis with no sample preparation required; D inherent high sensitivity; E real-time response; and F point sensing or standoff detection. LIBS sensor systems can be used to detect and analyze target samples by identifying all constituent elements and by determining either their relative or absolute abundances.
The revolutionary LIBS analyzer offers a number of benefits to Aluminum scrap metal recyclers and for incoming metal verification. The top three benefits include :
- The single second measurements making it a true ‘metal sorting’ system
- The fact that it does not have a radiation source so it limits the requirements of owners
- That it is extremely easy to use…it’s truly point, shoot, move to the next sample
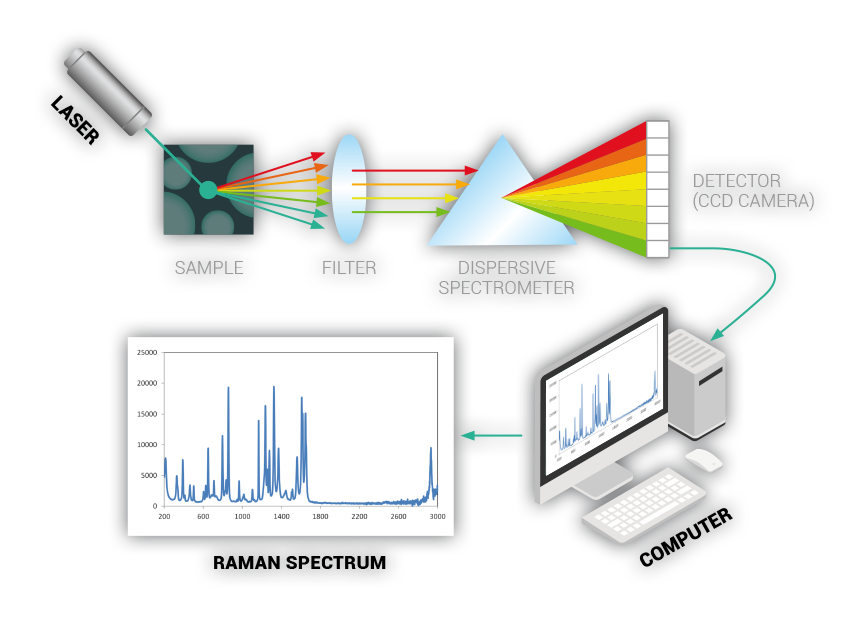
RAMAN TECHNOLOGY
Commonly used in chemistry to identify molecules
Raman spectroscopy is a spectroscopic technique used to observe vibrational, rotational, and other low-frequency modes in a system
Raman spectroscopy provides information about molecular vibrations that can be used for sample identification and quantitation. The technique involves shining a monochromatic light source (i.e. laser) on a sample and detecting the scattered light. The majority of the scattered light is of the same frequency as the excitation source; this is known as Rayleigh or elastic scattering.
A very small amount of the scattered light is shifted in energy from the laser frequency due to interactions between the incident electromagnetic waves and the vibrational energy levels of the molecules in the sample. Plotting the intensity of this "shifted" light versus frequency results in a Raman spectrum of the sample.